Moderna
W 2020 roku w związku ze światową. SAGE recommends the use of the Moderna mRNA-1273 vaccine at a schedule of two doses 100 µg 05 ml each 28 days apart.
Korzystając z tej witryny automatycznie akceptujesz używanie przez nas plików cookie.

Moderna. Modernas current vaccine shot is a 100-microgram dose compared with Pfizers 30-microgram dose. This Snapshot feature looks at the possible side effects and safety recommendations associated with this mRNA vaccine. Throughout the pandemic Ive always lumped Moderna and Pfizer together.
The Moderna COVID-19 Vaccine is a vaccine and may prevent you from getting COVID-19. Spikevax contains a molecule called messenger RNA mRNA with instructions for producing a protein from SARS-CoV-2 the virus that causes COVID-19. Modernas team recently showed that a half dose of the vaccine still sent antibody levels soaring.
There is no US. Moderna is a commercial-stage biotech that was founded in 2010 and had its initial public offering in December 2018. That may mean lighter side effects for Pfizers shot but in the long run the protection might not.
Food and Drug Administration to allow the use of a third booster dose of its Covid-19 vaccine. Based on those data the company asked the FDA. Cutting the Moderna doses in half could help reduce the risks of side effects from the booster.
This type of vaccine uses a genetic code called RNA to make your bodys cells produce the coronavirus specific spike protein. Your immune system cells then recognise the spike protein as a threat and begin building an immune response against it. Learn about safety data efficacy and clinical trial demographics.
Elasomeran codenamed mRNA-1273 and sold under the brand name Spikevax is a COVID-19 vaccine developed by American company Moderna the United States National Institute of Allergy and Infectious Diseases NIAID and the Biomedical Advanced Research and Development Authority BARDA. The additional shot significantly boosted immunity the. Moderna Therapeutics is pioneering a new class of drugs messenger RNA Therapeutics with the vast potential to treat many diseases across a range of drug modalities and therapeutic areas.
And BioNTech SE in research that compared immune responses evoked by. The Moderna COVID19 vaccine pINN. The FDA is considering booster shots of the Pfizer and BioNTech.
Spikevax is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. Kontaktmoderna-housepl Wiśniowa 6 21-040 Kalinówka Kontakt telefoniczny Poniedziałek - Piątek. Moderna submitted data from 344 volunteers who got a third shot of the vaccine six months after their first two doses.
Information about the Moderna COVID-19 vaccine including name manufacturer type of vaccine number of shots how it is given and links to ingredient information. Moderna on Wednesday asked the US. Amerykańskie przedsiębiorstwo biotechnologiczne z siedzibą w Cambridge w stanie Massachusetts założone w 2010 rokuPrzedsiębiorstwo zatrudnia 830 osób 2019.
This month to authorize 50 micrograms the. If necessary the interval between the doses may be extended to 42 days. Studies have shown a high public health impact where the interval has been longer than that recommended by the EUL.
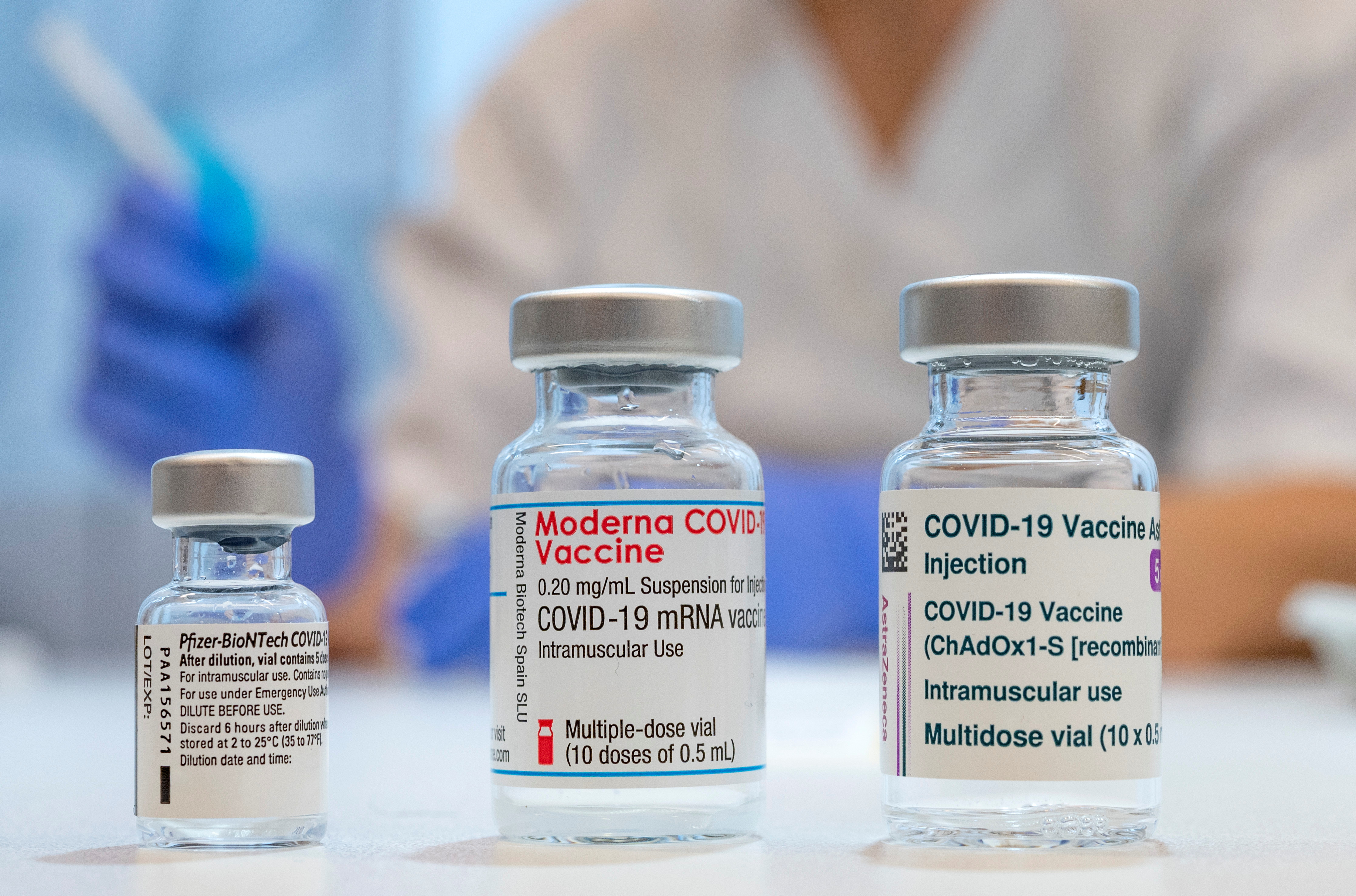
It is authorized for use in people. The Moderna vaccine is a messenger RNA mRNA vaccine. Food and Drug Administration FDA approved vaccine to prevent COVID-19.
Modernas shot consists of 100 micrograms of mRNA vaccine while Pfizers has 30 micrograms. Modernas mRNA based approach to creating vaccines works by enclosing a specific mRNA a piece of the viruss genetic information into an oily coating which releases the mRNA to the right site in the body. Moderna u osób które już chorowały na COVID-19.
The Moderna COVID-19 vaccine is a two-dose vaccine to prevent COVID-19. Moderna Incs Covid vaccine generated more than double the antibodies of a similar shot made by Pfizer Inc. Nie wiadomo jeszcze ile zaszczepionych osób może nadal.
Czy szczepionka COVID-19 Moderna może ograniczyć przenoszenie wirusa z jednej osoby na drugą. Wpływ szczepienia szczepionką OVID-19 Moderna na rozprzestrzenianie się wirusa SARS-CoV-2 w społeczności nie jest jeszcze znany. The Moderna Technology Center MTC is our innovation hub for Technical Development Manufacturing and Digital Excellence.
Działalność przedsiębiorstwa koncentruje się na odkrywaniu i opracowywaniu leków i technik szczepień opartych wyłącznie na informacyjnym RNA. The Moderna COVID-19 vaccine is an mRNA vaccine that requires 2 shots 28 days apart. With a fully digital cloud-based and highly automated site weve created an award-winning environmentally sustainable facility that supports the flexibility of our platform and helps accelerate development programs at.
800 - 1500 Niedziela. For example if there was evidence that Pfizer worked against a variant then Moderna. The mRNA chosen contains information about the viruss spike protein which is the target area for our antibodies to attack the virus.
800 - 1700 Sobota.
Sweden Halts Use Of Moderna S Covid Vaccine In Under 30s Cbs News
Moderna Plans African Vaccine Plant As Drugmakers Urged To Help Poorest Reuters
Why Moderna Stock Is Down By 15 Today
Sweden Denmark Pause Moderna Covid 19 Vaccine For Younger Age Groups
Sweden To Give 12 15 Year Olds Pfizer Vaccine Rejects Moderna Reuters
Allowing People To Mix Covid 19 Vaccines Could Cut Into Pfizer And Moderna S Revenue Next Year Marketwatch

Finland Also Limiting Moderna Vaccine For Younger Groups Thehill
Moderna Plans To Spend 500 Million On Vaccine Plant In Africa Bloomberg



/cloudfront-us-east-1.images.arcpublishing.com/gray/SKUFHPVFMNCM5OJ2V4G4FZKD3U.jpg)